solo per uso di ricerca
Dovitinib (TKI-258) FLT3 inibitore
N. Cat.S1018
Struttura chimica
Peso molecolare: 392.43
Controllo Qualità
| Target correlati | EGFR VEGFR JAK PDGFR FGFR Src HIF FLT HER2 Bcr-Abl |
|---|---|
| Altro FLT3 Inibitori | UNC2025 Crenolanib (CP-868596) Dovitinib (TKI258) Lactate monohydrate Tandutinib (MLN518) KW-2449 ENMD-2076 AST-487 (NVP-AST487) TCS 359 G-749 AMG 925 |
Coltura cellulare, trattamento e concentrazione di lavoro
| Linee cellulari | Tipo di saggio | Concentrazione | Tempo di incubazione | Formulazione | Descrizione dellattività | PMID |
|---|---|---|---|---|---|---|
| SupB15 | Growth Inhibition Assay | IC50=0.449 μM | 25202073 | |||
| SupB15-R | Growth Inhibition Assay | IC50=0.558 μM | 25202073 | |||
| BaF3-pSRα | Growth Inhibition Assay | IC50=0.668 μM | 25202073 | |||
| BaF3-p210Bcr-Abl | Growth Inhibition Assay | IC50=0.692 μM | 25202073 | |||
| BaF3-p210Bcr-Abl-T315I | Growth Inhibition Assay | IC50=2.626 μM | 25202073 | |||
| CCRF-CEM | Growth Inhibition Assay | IC50=0.398 μM | 25202072 | |||
| CEM/C2 | Growth Inhibition Assay | IC50=1.125 μM | 25202072 | |||
| Nalm-6 | Growth Inhibition Assay | IC50=0.382 μM | 25202072 | |||
| SEM-K2 | Growth Inhibition Assay | IC50=0.022 μM | 25202072 | |||
| HB-1119 | Growth Inhibition Assay | IC50=0.028 μM | 25202072 | |||
| RS4:11 | Growth Inhibition Assay | IC50=2.81 μM | 25202072 | |||
| Nalm-6 | Apoptosis Assay | 2 μM | 24/48 h | induces apoptosis resulting in about 72% of cell death after 24 h treatment and 81% after 48 h | 25202072 | |
| SEM-K2 | Apoptosis Assay | 0.1/1 μM | 24 h | induces early apoptosis of SEM-K2 cells at 0.1 μM after 24 h | 25202072 | |
| HCT-116 | Growth Inhibition Assay | IC50=3.050.58 μM | 24495750 | |||
| HT-29 | Growth Inhibition Assay | IC50=5.21.93 μM | 24495750 | |||
| SW-480 | Growth Inhibition Assay | IC50=4.330.47 μM | 24495750 | |||
| CaCO2 | Growth Inhibition Assay | IC50=3.230.64 μM | 24495750 | |||
| LS174T | Growth Inhibition Assay | IC50=4.330.47 μM | 24495750 | |||
| HEC-1A | Function Assay | 0.05/0.1/0.5 μM | 72 h | causes a decrease in STAT3, ERK, and AKT phosphorylation | 24495750 | |
| AN3CA | Function Assay | 0.05/0.1/0.5 μM | 72 h | causes a decrease in STAT3, ERK, and AKT phosphorylation | 24495750 | |
| MFE-296 | Function Assay | 0.05/0.1/0.5 μM | 72 h | causes a decrease in STAT3, ERK, and AKT phosphorylation | 24495750 | |
| UMC3 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| 5637 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| HU456 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| MGHU4 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| HT1376 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| RT112 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| T24 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| BFTC905 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| TCC-SUP | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| RT4 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| HONE1 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| HNE1 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| CNE2 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| C666-1 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| HeLa | Growth Inhibition Assay | 0.1-10 μM | 24 h | induces G2/M arrest in a concentration-dependent manner | 24238094 | |
| Hep3B | Growth Inhibition Assay | 0.1-10 μM | 24 h | induces G2 arrest | 24238094 | |
| HepG2 | Growth Inhibition Assay | 48 h | IC50=2.727 ± 0.429 μM | 23546591 | ||
| Hep3B | Growth Inhibition Assay | 48 h | IC50=4.223 ± 0.839 μM | 23546591 | ||
| PLC/PRF5 | Growth Inhibition Assay | 48 h | IC50=16.120 ± 4.001 μM | 23546591 | ||
| Huh7 | Growth Inhibition Assay | 48 h | IC50=15.007 ± 7.334 μM | 23546591 | ||
| HepG2 | Growth Inhibition Assay | 72 h | IC50=1.200 ± 0.226 μM | 23546591 | ||
| Hep3B | Growth Inhibition Assay | 72 h | IC50=0.892 ± 0.044 μM | 23546591 | ||
| PLC/PRF5 | Growth Inhibition Assay | 72 h | IC50=3.110 ± 0.337 μM | 23546591 | ||
| Huh7 | Growth Inhibition Assay | 72 h | IC50=3.980 ± 0.803 μM | 23546591 | ||
| MFE280 | Growth Inhibition Assay | IC50=0.42 ± 0.06 μM | 23443805 | |||
| AN3CA | Growth Inhibition Assay | IC50=0.50 ± 0.10 μM | 23443805 | |||
| HEC155 | Growth Inhibition Assay | IC50=0.66 ± 0.09 μM | 23443805 | |||
| MFE296 | Growth Inhibition Assay | IC50=0.66 ± 0.19 μM | 23443805 | |||
| SPAC1S | Growth Inhibition Assay | IC50=0.77 ± 0.08 μM | 23443805 | |||
| RL952 | Growth Inhibition Assay | IC50=0.93 ± 0.01 μM | 23443805 | |||
| EN1 | Growth Inhibition Assay | IC50=1.02 ± 0.25 μM | 23443805 | |||
| SNGII | Growth Inhibition Assay | IC50=1.24 ± 0.28 μM | 23443805 | |||
| ISHIKAWA | Growth Inhibition Assay | IC50=1.30 ± 0.11 μM | 23443805 | |||
| HEC1A | Growth Inhibition Assay | IC50=1.34 ± 0.30 μM | 23443805 | |||
| KLE | Growth Inhibition Assay | IC50=1.37 ± 0.02 μM | 23443805 | |||
| SNGM | Growth Inhibition Assay | IC50=1.42 ± 0.13 μM | 23443805 | |||
| USPC2 | Growth Inhibition Assay | IC50=1.62 ± 0.01 μM | 23443805 | |||
| EN | Growth Inhibition Assay | IC50=1.66 ± 0.01 μM | 23443805 | |||
| MFE319 | Growth Inhibition Assay | IC50=1.87 ± 0.45 μM | 23443805 | |||
| EFE184 | Growth Inhibition Assay | IC50=2.04 ± 0.13 μM | 23443805 | |||
| ECC1 | Growth Inhibition Assay | IC50=2.07 ± 0.01 μM | 23443805 | |||
| HEC1B | Growth Inhibition Assay | IC50=2.57 ± 0.23 μM | 23443805 | |||
| USPC1 | Growth Inhibition Assay | IC50=2.60 ± 0.13 μM | 23443805 | |||
| SPAC1L | Growth Inhibition Assay | IC50=3.06 ± 1.14 μM | 23443805 | |||
| HUVEC | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| HMVEC | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| MHCC-97H | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| SMMC7721 | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| Huh-7 | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| Sk-Hep1 | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| Hep3B | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| PLC5 | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| PLC5 | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| Hep3B | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| Sk-Hep1 | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| Huh-7 | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| PLC5 | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| Hep3B | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| Sk-Hep1 | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| Huh-7 | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| PLC5 | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| Hep3B | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| Sk-Hep1 | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| Huh-7 | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| SW780 | Growth Inhibition Assay | 5 d | IC50=50 nM | 21119661 | ||
| RT112 | Growth Inhibition Assay | 5 d | IC50=15 nM | 21119661 | ||
| RT4 | Growth Inhibition Assay | 5 d | IC50=5 nM | 21119661 | ||
| JMSU1 | Growth Inhibition Assay | 5 d | IC50=50 nM | 21119661 | ||
| J82 | Growth Inhibition Assay | 5 d | IC50=1400 nM | 21119661 | ||
| 97-7 | Growth Inhibition Assay | 5 d | IC50=1000 nM | 21119661 | ||
| RT112 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| RT4 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| MGH-U3 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| SW780 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| 97-7 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| J807C | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| Y373C | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| K650E | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| G384D | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| F384L | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| KMS11 | Growth Inhibition Assay | 72 h | IC50=90 nM | 15598814 | ||
| KMS18 | Growth Inhibition Assay | 72 h | IC50=550 nM | 15598814 | ||
| OPM2 | Growth Inhibition Assay | 72 h | IC50=90 nM | 15598814 | ||
| H929 | Growth Inhibition Assay | 72 h | IC50> 2500 nM | 15598814 | ||
| 8226 | Growth Inhibition Assay | 72 h | IC50> 2500 nM | 15598814 | ||
| U266 | Growth Inhibition Assay | 72 h | IC50> 2500 nM | 15598814 | ||
| KM12L4A | Function assay | Inhibition of VEGFR2 phosphorylation expressed in human KM12L4A cells by Western blot analysis, EC50=0.046μM | 19113866 | |||
| KM12L4A | Function assay | Inhibition of PDGFRbeta phosphorylation expressed in human KM12L4A cells Western blot analysis, EC50=0.051μM | 19113866 | |||
| KM12L4A | Function assay | Inhibition of FGFR1 phosphorylation expressed in human KM12L4A cells by Western blot analysis, EC50=0.166μM | 19113866 | |||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant PDGFRbeta (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGLFDDPSYVNVQNL in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.001μM | 27914362 | ||
| Sf9 | Function assay | 1 to 4 hrs | Inhibition of recombinant human N-terminal GST/His6-tagged c-KIT (544 to 976 residues) expressed in baculovirus infected sf9 cells using biotinylated peptide substrate GGLFDDPSYVNVQNL in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescen, IC50=0.001μM | 27914362 | ||
| Sf9 | Function assay | 1 to 4 hrs | Inhibition of recombinant human N-terminal GST/His6-tagged FLT3 (571 to 993 residues) expressed in baculovirus infected sf9 cells using biotinylated peptide substrate GGLFDDPSYVNVQNL in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescenc, IC50=0.001μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant FGFR1 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.008μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant VEGFR3 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.008μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant VEGFR1 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.01μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant VEGFR2 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.013μM | 27914362 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant FGFR1 (unknown origin) expressed in baculovirus infected insect cells using GGGGQDGKDYIVLPI as substrate after 1 to 4 hrs by time-resolved fluorescence assay, IC50=0.008μM | 30503938 | ||
| NCI-H1703 | Function assay | 10 uM | 24 hrs | Inhibition of TNIK in human NCI-H1703 cells transfected with lentiviral vector 7TFP assessed as reduction of GSK3 inhibitor X activated TNIK-mediated Wnt/TCF/beta-catenin-dependent transcription at 10 uM after 24 hrs by luciferase reporter assay | ChEMBL | |
| LoVo | Cytotoxicity assay | 10 uM | 72 hrs | Cytotoxicity against Wnt/beta-catenin signalling dependent human LoVo cells assessed as cell viability at 10 uM after 72 hrs by ATPlite assay | ChEMBL | |
| HCT116 | Cytotoxicity assay | 10 uM | 72 hrs | Cytotoxicity against Wnt/beta-catenin signalling dependent human HCT116 cells assessed as cell viability at 10 uM after 72 hrs by ATPlite assay | ChEMBL | |
| Clicca per visualizzare più dati sperimentali sulle linee cellulari | ||||||
Informazioni chimiche, conservazione e stabilità
| Peso molecolare | 392.43 | Formula | C21H21FN6O |
Conservazione (Dalla data di ricezione) | |
|---|---|---|---|---|---|
| N. CAS | 405169-16-6 | Scarica SDF | Conservazione delle soluzioni stock |
|
|
| Sinonimi | CHIR-258 | Smiles | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=C(C5=C(C=CC=C5F)NC4=O)N | ||
Solubilità
|
In vitro |
DMSO
: 30 mg/mL
(76.44 mM)
Water : Insoluble Ethanol : Insoluble |
Calcolatore di Molarità
|
In vivo |
|||||
Calcolatore di formulazione in vivo (Soluzione chiara)
Passo 1: Inserire le informazioni di seguito (Consigliato: Un animale aggiuntivo per tenere conto della perdita durante lesperimento)
Passo 2: Inserire la formulazione in vivo (Questo è solo il calcolatore, non la formulazione. Contattateci prima se non cè una formulazione in vivo nella sezione Solubilità.)
Risultati del calcolo:
Concentrazione di lavoro: mg/ml;
Metodo per preparare il liquido master di DMSO: mg farmaco predissolto in μL DMSO ( Concentrazione del liquido master mg/mL, Vi preghiamo di contattarci prima se la concentrazione supera la solubilità del DMSO del lotto del farmaco. )
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungereμL PEG300, mescolare e chiarire, quindi aggiungereμL Tween 80, mescolare e chiarire, quindi aggiungere μL ddH2O, mescolare e chiarire.
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungere μL Olio di mais, mescolare e chiarire.
Nota: 1. Si prega di assicurarsi che il liquido sia limpido prima di aggiungere il solvente successivo.
2. Assicurarsi di aggiungere il/i solvente/i in ordine. È necessario assicurarsi che la soluzione ottenuta, nellaggiunta precedente, sia una soluzione limpida prima di procedere allaggiunta del solvente successivo. Metodi fisici come il vortex, gli ultrasuoni o il bagno dacqua calda possono essere utilizzati per facilitare la dissoluzione.
Meccanismo dazione
| Targets/IC50/Ki |
FLT3
(Cell-free assay) 1 nM
c-Kit
(Cell-free assay) 2 nM
FGFR1
(Cell-free assay) 8 nM
VEGFR3/FLT4
(Cell-free assay) 8 nM
FGFR3
(Cell-free assay) 9 nM
VEGFR1/FLT1
(Cell-free assay) 10 nM
VEGFR2/Flk1
(Cell-free assay) 13 nM
PDGFRβ
(Cell-free assay) 27 nM
CSF-1R/c-Fms
(Cell-free assay) 36 nM
|
|---|---|
| In vitro |
Dovitinib (TKI-258) inibisce potentemente la crescita stimolata da FGF delle cellule B9 che esprimono WT e F384L-FGFR3 con IC50 di 25 nM. Inoltre, inibisce la proliferazione delle cellule B9 che esprimono ciascuna delle diverse mutanti attivate di FGFR3. È interessante notare che si osservano differenze minime nella sensibilità delle diverse mutazioni di FGFR3 a questo composto, con l'IC50 che varia da 70 a 90 nM per ciascuna delle diverse mutazioni. Le cellule B9 dipendenti dall'IL-6 contenenti solo il vettore (cellule B9-MINV) sono resistenti alla sua attività inibitoria a concentrazioni fino a 1 µM. Inibisce la proliferazione cellulare delle cellule KMS11 (FGFR3-Y373C), OPM2 (FGFR3-K650E) e KMS18 (FGFR3-G384D) con IC50 di 90 nM (KMS11 e OPM2) e 550 nM, rispettivamente. Il composto inibisce la fosforilazione di ERK1/2 mediata da FGF e induce citotossicità nelle cellule MM primarie che esprimono FGFR3. I BMSC conferiscono un modesto grado di resistenza con un'inibizione della crescita del 44,6% per le cellule trattate con 500 nM di Dovitinib e coltivate su stroma rispetto a un'inibizione della crescita del 71,6% per le cellule cresciute senza BMSC. Inibisce la proliferazione di M-NFS-60, una linea cellulare mieloblastica murina guidata dalla crescita di M-CSF con una concentrazione efficace mediana (EC50) di 220 nM. Il trattamento delle cellule SK-HEP1 con questo composto si traduce in una riduzione dose-dipendente del numero di cellule e un arresto in fase G2/M con riduzione delle fasi G0/G1 e S, inibizione della crescita indipendente dall'ancoraggio e blocco della motilità cellulare indotta da bFGF. L'IC50 per questo composto nelle cellule SK-HEP1 è di circa 1,7 µM. Riduce anche significativamente i livelli basali di fosforilazione di FGFR-1, del substrato 2α di FGFR (FRS2-α) e di ERK1/2 ma non di Akt sia nelle cellule SK-HEP1 che nelle 21-0208. Nelle cellule HCC 21-0208, inibisce significativamente la fosforilazione indotta da bFGF di FGFR-1, FRS2-α, ERK1/2 ma non di Akt. |
| Saggio chinasico |
Saggi chinasici in vitro
|
|
I valori della concentrazione inibitoria del 50% (IC50) per l'inibizione delle RTK da parte di Dovitinib (TKI-258) sono determinati in un formato di fluorescenza risolta nel tempo (TRF) o radioattivo, misurando la sua inibizione del trasferimento di fosfato a un substrato da parte del rispettivo enzima. I domini chinasici di FGFR3, FGFR1, PDGFRβ e VEGFR1-3 sono saggiati in 50 mM HEPES (acido N-2-idrossietilpiperazina-N′-2-etansolfonico), pH 7,0, 2 mM MgCl2, 10 mM MnCl2, 1 mM NaF, 1 mM ditiotreitolo (DTT), 1 mg/mL di albumina sierica bovina (BSA), 0,25 μM di substrato peptidico biotinilato (GGGGQDGKDYIVLPI) e da 1 a 30 μM di adenosina trifosfato (ATP) a seconda del Km per il rispettivo enzima. Le concentrazioni di ATP sono a o appena al di sotto del Km. Per le reazioni di c-KIT e FLT3 il pH viene aumentato a 7,5 con 0,2-8 μM di ATP in presenza di 0,25-1 μM di substrato peptidico biotinilato (GGLFDDPSYVNVQNL). Le reazioni sono incubate a temperatura ambiente per 1-4 ore e il peptide fosforilato viene catturato su piastre microtiter rivestite di streptavidina contenenti tampone di blocco della reazione (25 mM EDTA [acido etilendiamminotetraacetico], 50 mM HEPES, pH 7,5). Il peptide fosforilato viene misurato con il sistema DELFIA TRF utilizzando un anticorpo anti-fosfotirosina PT66 marcato con Europio. La concentrazione di questo composto per l'IC50 è calcolata utilizzando la regressione non lineare con il software di analisi dati XL-Fit versione 4.1 (IDBS). L'inibizione dell'attività chinasica del recettore del fattore stimolante le colonie-1 (CSF-1R), PDGFRα, recettore dell'insulina (InsR) e recettore del fattore di crescita insulino-simile 1 (IGFR1) è determinata a concentrazioni di ATP vicine al Km per l'ATP.
|
|
| In vivo |
Dovitinib (TKI-258) induce risposte sia citostatiche che citotossiche in vivo, con conseguente regressione dei tumori che esprimono FGFR3. Mostra un'inibizione dose- e esposizione-dipendente dei recettori tirosin chinasici (RTK) bersaglio espressi negli xenotrapianti tumorali. Questo composto inibisce potentemente la crescita tumorale di sei linee di HCC. L'inibizione dell'Angiogenesis è correlata all'inattivazione delle vie di segnalazione FGFR/PDGFRβ/VEGFR2. In un modello ortotopico, inibisce potentemente la crescita tumorale primaria e le metastasi polmonari e prolunga significativamente la sopravvivenza dei topi. La somministrazione di Dovitinib si traduce in una significativa inibizione della crescita tumorale e regressioni tumorali, compresi tumori grandi e consolidati (500-1.000 mm3). |
Riferimenti |
|
Applicazioni
| Metodi | Biomarcatori | Immagini | PMID |
|---|---|---|---|
| Western blot | CDK1 / p-CDK1 / p53 / p21 p-PDGFR-β / PDGFR-β / p-ERK / ERK p-VEGFR-2 / VEGFR-2 / p-FGFR-1 / FGFR-1 p-STAT3 / STAT3 / Mcl-1 / LC3 / Beclin 1 / p62 |
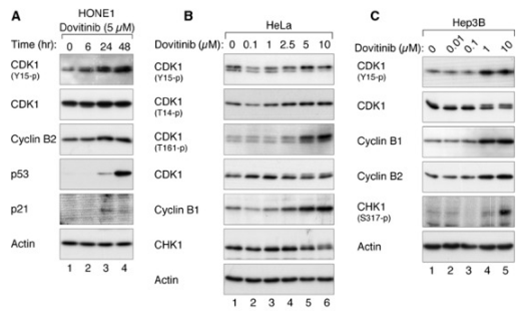
|
24238094 |
| Growth inhibition assay | Cell viability |
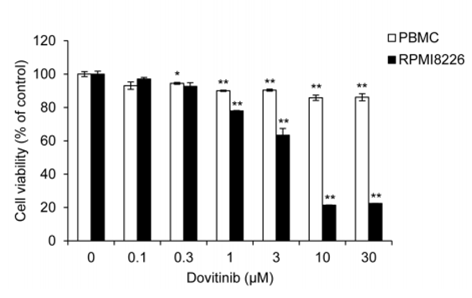
|
28467797 |
Informazioni sullo studio clinico
(dati da https://clinicaltrials.gov, aggiornato il 2024-05-22)
| Numero NCT | Reclutamento | Condizioni | Sponsor/Collaboratori | Data di inizio | Fasi |
|---|---|---|---|---|---|
| NCT05571969 | Recruiting | Advanced Solid Tumors |
Allarity Therapeutics|Amarex Clinical Research |
February 20 2023 | Phase 1 |
| NCT02268435 | Withdrawn | Gastrointestinal Stromal Tumors |
Asan Medical Center |
March 2015 | Phase 1 |
| NCT01700270 | Completed | Advanced Solid Tumors Excluding Breast Cancer |
Novartis Pharmaceuticals|Novartis |
May 2013 | Phase 1 |
| NCT01680796 | Withdrawn | Multiple Myeloma |
University of Florida|Novartis Pharmaceuticals |
February 2013 | Phase 1 |
| NCT01266070 | Terminated | Von Hippel-Lindau Syndrome |
M.D. Anderson Cancer Center|Novartis |
November 2012 | Phase 2 |
Supporto tecnico
Istruzioni per la manipolazione
Tel: +1-832-582-8158 Ext:3
Per qualsiasi altra domanda, si prega di lasciare un messaggio.
