solo per uso di ricerca
Necrostatin-1 (Nec-1) Inibitore di RIPK1
N. Cat.S8037
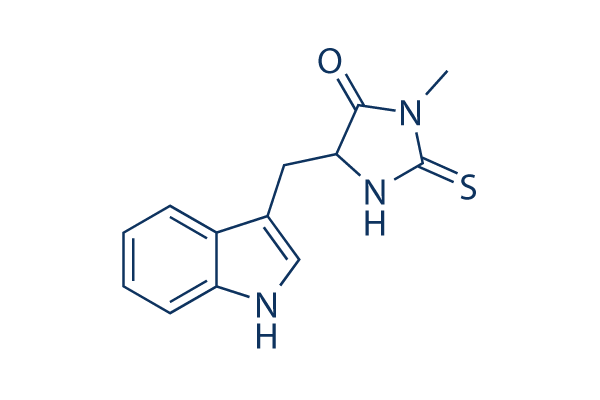
Struttura chimica
Peso molecolare: 259.33
Controllo Qualità
| Target correlati | Bcl-2 Caspase PD-1/PD-L1 Ferroptosis p53 Apoptosis related Synthetic Lethality STAT TNF-alpha Ras |
|---|---|
| Altro RIP kinase Inibitori | Necrostatin 2 racemate (Nec-1s) GSK872 Mito-TEMPO GSK'963 RIPA-56 GSK2982772 Resibufogenin GSK583 HS-1371 GSK2983559 (compound 3) |
Coltura cellulare, trattamento e concentrazione di lavoro
| Linee cellulari | Tipo di saggio | Concentrazione | Tempo di incubazione | Formulazione | Descrizione dellattività | PMID |
|---|---|---|---|---|---|---|
| HT-22 | Cytotoxicity Assay | 10 μM | 12 h | protects against cell death induced by 5 mmol/L glutamate | 17760869 | |
| Jurkat | Function Assay | 200 μm | 30 min | reduces Naegleria fowleri-induced reactive oxygen species (ROS) generation | 21535020 | |
| Jurkat | Cytotoxicity Assay | 50/ 100/200 μm | 1/3 h | reduces Naegleria fowleri-induced cytotoxicity | 21535020 | |
| SW13 | Cell Viability Assay | 100 μM | 24 h | DMSO | increases cellular survival | 22136818 |
| 8505c | Cell Viability Assay | 100 μM | 24 h | DMSO | increases cellular survival | 22136818 |
| TPC-1 | Cell Viability Assay | 100 μM | 24 h | DMSO | increases cellular survival | 22136818 |
| L929sA | Apoptosis Assay | 10 μM | 1 h | abrogates the interaction of caspase-8 with FADD | 22362767 | |
| L929sA | Apoptosis Assay | 10 μM | 1 h | rescues cells expressing RIPK1ΔID from TNF-induced apoptosis | 22362767 | |
| L929sA | Apoptosis Assay | 10 μM | 1 h | inhibits the apoptotic response to TNF | 22362767 | |
| K562/Adr | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| K562 | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| HL60/Adr | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| HL60 | Function Assay | 60 μM | 12 h | increases the activity of caspases, caspase 8 and 9 | 22837689 | |
| K562/Adr | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| K562 | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| HL60/Adr | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| HL60 | Function Assay | 60 μM | 12 h | augments the caspase-3 activity | 22837689 | |
| K562/Adr | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| K562 | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| HL60/Adr | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| HL60 | Apoptosis Assay | 60 μM | 12 h | enhances shikonin-induced apoptosis | 22837689 | |
| SH-EP | Apoptosis Assay | 10 μM | 72 h | inhibits IAP inhibitor- and Lexatumumab-induced apoptosis | 22890322 | |
| NIH3T3 | Function Assay | 10/50 μM | 1/3 h | ameliorates TNFα-driven complex formation | 23261677 | |
| HT-22 | Function Assay | 25 μM | 0–30 min | DMSO | inhibits ERK Activation induced by glutamate | 23307752 |
| HT-22 | Cell Viability Assay | 10 μM | 12 h | DMSO | protects against glutamate-induced cell death | 23307752 |
| KMS-12-BM | Cytotoxicity Assay | 90 µM | 1 h | blocks BAY 11-7082 induced rapid cell swelling | 23527154 | |
| MM.1S | Cytotoxicity Assay | 90 µM | 1 h | blocks BAY 11-7082 induced rapid cell swelling | 23527154 | |
| ΔN-Karpas 299 | Cytotoxicity Assay | 20 μM | 16 h | inhibits CD30-induced cell death | 23545938 | |
| MEFs | Function Assay | 20 μM | 1/2/4 h | suppresses TNFα-induced RIPK1 phosphorylation | 23727581 | |
| MEFs | Cytotoxicity Assay | 2/6/20 μM | 18 h | inhibits TNFα-induced cell death in RelA KO MEFs | 23727581 | |
| RMS13 | Cell Viability Assay | 40 μg/ml | 24 h | rescues GX15-070-induced loss of cell viability | 23744296 | |
| TE671 | Cell Viability Assay | 40 μg/ml | 24 h | rescues GX15-070-induced loss of cell viability | 23744296 | |
| U87 | Function Assay | 1 mmol/L | 1.5-3 h | suppresses the expression of RIP-1 caused by shikonin | 23840441 | |
| C6 | Function Assay | 1 mmol/L | 1.5-3 h | suppresses the expression of RIP-1 caused by shikonin | 23840441 | |
| U87 | Cytotoxicity Assay | 1 mmol/L | 3 h | blocks shikonin induced necrosis | 23840441 | |
| C6 | Cytotoxicity Assay | 1 mmol/L | 3 h | blocks shikonin induced necrosis | 23840441 | |
| U87 | Cell Viability Assay | 1 mmol/L | 3 h | attenuates Shikonin induced glioma cell death | 23840441 | |
| C6 | Cell Viability Assay | 1 mmol/L | 3 h | attenuates Shikonin induced glioma cell death | 23840441 | |
| L929 | Function Assay | 5 μg/ml | 24 h | blocks zVAD induced necroptosis and autophagy | 23941769 | |
| L929 | Function Assay | 2 μg/ml | 24 h | promots caspase-6 (p20) activity and procaspase-6 cleavage | 23941769 | |
| L929 | Growth Inhibition Assay | 2/5 μg/ml | 24 h | reverses the cell growth inhibition and cell death induced by TNFα alone as well as TNFα + zVAD | 23941769 | |
| L929 | Function Assay | 2/5 μg/ml | 24 h | reversed the autophagy induced by TNFα alone as well as TNFα + zVAD | 23941769 | |
| NRK-52E | Cell Viability Assay | 20 μM | 24 h | inhibits increased Drp1 protein expression after TNF-α Stimulation and ATP Depletion | 24351845 | |
| NRK-52E | Cell Viability Assay | 20 μM | 24 h | increases cell viability after TNF-α Stimulation and ATP Depletion | 24351845 | |
| NRK-52E | Cell Viability Assay | 20 μM | 24 h | protects cells from cell death caused by ischemia injury | 24351845 | |
| AGS | Cell Viability Assay | 60 μm | 1 h | prevents shikonin-induced cell death | 24463199 | |
| L-540 | Cell Viability Assay | 60 μm | 1 h | reduces the Givinostat/Sorafenib-induced cell death | 24561519 | |
| L-540 | Function Assay | 60 μm | 1 h | prevents the mitochondrial membrane depolarization | 24561519 | |
| L-540 | Function Assay | 60 μm | 1 h | prevents the generation of ROS | 24561519 | |
| SK-Hep1 | Function Assay | 60 μM | 18 h | blocks β-lapachone-mediated PAR accumulation and AIF translocation to the cytosol | 24832602 | |
| SK-Hep1 | Function Assay | 60 μM | 18 h | inhibits β-Lapachone-induced leakage of HMGB-1 | 24832602 | |
| SK-Hep1 | Function Assay | 60 μM | 18 h | blocks β-lapachone-induced morphological change, cell death and PI uptake | 24832602 | |
| OHC | Function Assay | 300 μM | DMSO | increases the number of apoptotic OHCs without altering the levels of CC8 after noise exposure | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | diminishes noise-induced AMPK activation | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | results in a reduction of noise-induced RIP1 and RIP3 immunofluorescence | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | decreases noise-induced swollen nuclei | 24874734 | |
| OHC | Function Assay | 300 μM | DMSO | increases noise-induced condensed nuclei | 24874734 | |
| Huh7 | Cell Viability Assay | 50 µM | 24/48 h | DMSO | prevents cell death of rAdHCV co-infected Huh7 cells | 24973240 |
| L929 | Cell Viability Assay | 30 μM | 1 h | inhibits TNF-α-induced cleavage of Topo I | 25095742 | |
| L929 | Cell Viability Assay | 30 μM | 1 h | inhibits TNF-α-induced loss of cell viability | 25095742 | |
| L929-A | Function Assay | 50 μM | 12 h | inhibits the TNFα-induced loss of mitochondrial membrane permeability | 25398540 | |
| L929 | Function Assay | 50 μM | 12 h | inhibits TNFα-induced Bid cleavage | 25398540 | |
| L929-N | Function Assay | 50 μM | 12 h | blocks the cleavage of Caspase-3 and PARP | 25398540 | |
| L929-A | Function Assay | 50 μM | 12 h | blocks the cleavage of Caspase-3 and PARP | 25398540 | |
| L929-N | Cell Viability Assay | 50 μM | 24 h | blocks TNFα-induced cell death | 25398540 | |
| L929-A | Cell Viability Assay | 50 μM | 24 h | blocks TNFα-induced cell death | 25398540 | |
| KMS-12-PE | Cell Viability Assay | 60 μM | 5 h | inhibits SHK-induced cell death | 25530098 | |
| SGC-7901 | Cell Viability Assay | 30 μM | 1 h | suppresses oxaliplatin-mediated cell death | 25767076 | |
| BxPC-3 | Function Assay | 20 μM | 24 h | decreases the early necrotic cells | 26000607 | |
| MiaPaCa-2 | Function Assay | 20 μM | 24 h | decreases the early necrotic cells | 26000607 | |
| NCI-H28 | Cell Viability Assay | 10 μM | 24 h | prevents DAPE-induced reduction of NCI-H28 cell viability | 26004138 | |
| BMDM | Function Assay | 10 μM | 30 min | protects cells from TAKI-induced LDH release | 26381601 | |
| MEFs | Cell Viability Assay | 10 μM | 48 h | DMSO | inhibits zVAD-promoted death of CNOT3-depleted MEFs | 26437789 |
| A549 | Cell Viability Assay | 50 μM | 24 h | inhibits MMS-induced cell death | 26472723 | |
| Jurkat T | Necroptosis assay | Inhibition of TNF-alpha-induced necroptosis in FADD-deficient human Jurkat T cells, EC50 = 0.05 μM. | 18467094 | |||
| Jurkat | Function assay | Inhibition of endogenous RIP1 autophosphorylation in human Jurkat cells, EC50 = 0.182 μM. | 18408713 | |||
| Sf9 | Function assay | 30 mins | Inhibition of recombinant human GST-fused RIPK1 (1 to 497 residues) expressed in baculovirus infected insect Sf9 cells in presence of 32P-gamma-ATP after 30 mins by autoradiogram-based Western blot method, IC50 = 0.182 μM. | 28280261 | ||
| Jurkat T | Necroptosis assay | Effective concentration required for inhibition of necroptosis in FADD deficient Jurkat T cells treated with TNF-alpha, EC50 = 0.49 μM. | 16153840 | |||
| Jurkat | Necroptosis assay | Inhibition of cellular necroptosis in TNFalpha treated FADD deficient human Jurkat cells, EC50 = 0.49 μM. | 18408713 | |||
| Jurkat T | Necroptosis assay | 30 uM | 24 hrs | Inhibition of necroptosis in TNF-alpha-induced human Jurkat T cells assessed as cell viability at 30 uM after 24 hrs | 18467094 | |
| L929 | Necroptosis assay | 30 uM | 24 hrs | Inhibition of necroptosis in zVAD-induced mouse L929 cells assessed as cell viability at 30 uM after 24 hrs | 18467094 | |
| L929 | Necroptosis assay | 30 uM | 24 hrs | Inhibition of necroptosis in TNF-alpha-induced mouse L929 cells assessed as cell viability at 30 uM after 24 hrs | 18467094 | |
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necrotic cell death in mouse 3T3 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of TNFalpha and zVAD.fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necrotic cell death in mouse 3T3 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of FasL and zVAD.fmk | 16408008 | ||
| MEF | Cell death assay | 16 hrs | Inhibition of death receptor signaling mediated necrotic cell death in SV40 transformed mouse MEF cells assessed as cell viability after 16 hrs by ATP based viability assay in presence of TNFalpha, CHX and zVAD.fmk | 16408008 | ||
| IEC18 | Cell death assay | Inhibition of death receptor signaling mediated necrotic cell death in rat IEC18 cells assessed as cell viability in presence of TNFalpha and zVAD.fmk | 16408008 | |||
| HL60 | Cell death assay | Inhibition of death receptor signaling mediated necrotic cell death in human HL60 cells assessed as cell viability in presence of TNFalpha and zVAD.fmk | 16408008 | |||
| L929 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necrotic cell death in mouse L929 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of TNFalpha | 16408008 | ||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as nuclear condensation by bright field microscopy in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as organelle swelling by bright field microscopy in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as early loss of plasma membrane integrity by bright field microscopy in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells assessed as appearance of translucent cytosol in presence of FasL, CHX and zVAD-fmk | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of nuclear condensation by bright field microscopy in presence of TNFalpha | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of organelle swelling by bright field microscopy in presence of TNFalpha | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of early loss of plasma membrane integrity by bright field microscopy in presence of TNFalpha | 16408008 | |||
| Jurkat | Necrosis assay | Inhibition of necrosis in human Jurkat cells deficient in FADD assessed as inhibition of appearance of translucent cytosol in presence of TNFalpha | 16408008 | |||
| U937 | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human U937 cells assessed as cell viability after 48 hrs by ATP based viability assay in presence of TNFalpha and zVAD-fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as cell viability after 24 hrs by ATP based viability assay in presence of TNFalpha and zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD assessed as decreased levels of PE-conjugated LC3-II (autophagy marker) after 24 hrs by Western blot method in presence of TNFalpha | 16408008 | ||
| L929 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse L929 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of TNFalpha | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of TNFalpha and zVAD-fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of FasL and zVAD-fmk | 16408008 | ||
| 3T3 | Cell death assay | 24 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in mouse 3T3 cells assessed as decreased levels of PE-conjugated autophagy marker LC3-II after 24 hrs by Western blot method in presence of rapamycin | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing FKBP12-based dimerization domain assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP K45M mutant assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase domain assessed as cell viability after 48 hrs by FACS in presence of AP1510, zVAD-fmk | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing FKBP12-based dimerization domain assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP K45M mutant assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Jurkat | Cell death assay | 48 hrs | Inhibition of death receptor signaling mediated necroptotic cell death in human Jurkat cells deficient in FADD and expressing RIP kinase domain assessed as cell viability after 48 hrs by FACS in presence of AP1510 | 16408008 | ||
| Sf9 | Function assay | Inhibition of human RIP1 K45M mutant autophosphorylation expressed in Sf9 cells | 18408713 | |||
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against FasL-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by FasL stimulation measured after 20 hrs by Alamar blue assay | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against CHX-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by CHX stimulation by Alamar blue assay | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against Z-VAD-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by Z-VAD stimulation by Alamar blue assay | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against FasL-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by FasL stimulation measured after 20 hrs by phase contrast microscopy | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against CHX-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by CHX stimulation by phase contrast microscopy | 29541357 | |
| Jurkat | Cytoprotective assay | 30 uM | 1 hr | Cytoprotective activity against Z-VAD-induced necroptosis in human Jurkat cells assessed as increase in cell viability at 30 uM incubated for 1 hr followed by Z-VAD stimulation by phase contrast microscopy | 29541357 | |
| Clicca per visualizzare più dati sperimentali sulle linee cellulari | ||||||
Informazioni chimiche, conservazione e stabilità
| Peso molecolare | 259.33 | Formula | C13H13N3OS |
Conservazione (Dalla data di ricezione) | |
|---|---|---|---|---|---|
| N. CAS | 4311-88-0 | Scarica SDF | Conservazione delle soluzioni stock |
|
|
| Sinonimi | Nec-1 | Smiles | CN1C(=O)C(NC1=S)CC2=CNC3=CC=CC=C32 | ||
Solubilità
|
In vitro |
DMSO
: 57 mg/mL
(219.79 mM)
Water : Insoluble Ethanol : Insoluble |
Calcolatore di Molarità
|
In vivo |
|||||
Calcolatore di formulazione in vivo (Soluzione chiara)
Passo 1: Inserire le informazioni di seguito (Consigliato: Un animale aggiuntivo per tenere conto della perdita durante lesperimento)
Passo 2: Inserire la formulazione in vivo (Questo è solo il calcolatore, non la formulazione. Contattateci prima se non cè una formulazione in vivo nella sezione Solubilità.)
Risultati del calcolo:
Concentrazione di lavoro: mg/ml;
Metodo per preparare il liquido master di DMSO: mg farmaco predissolto in μL DMSO ( Concentrazione del liquido master mg/mL, Vi preghiamo di contattarci prima se la concentrazione supera la solubilità del DMSO del lotto del farmaco. )
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungereμL PEG300, mescolare e chiarire, quindi aggiungereμL Tween 80, mescolare e chiarire, quindi aggiungere μL ddH2O, mescolare e chiarire.
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungere μL Olio di mais, mescolare e chiarire.
Nota: 1. Si prega di assicurarsi che il liquido sia limpido prima di aggiungere il solvente successivo.
2. Assicurarsi di aggiungere il/i solvente/i in ordine. È necessario assicurarsi che la soluzione ottenuta, nellaggiunta precedente, sia una soluzione limpida prima di procedere allaggiunta del solvente successivo. Metodi fisici come il vortex, gli ultrasuoni o il bagno dacqua calda possono essere utilizzati per facilitare la dissoluzione.
Meccanismo dazione
| Caratteristiche |
A powerful tool for characterizing the role of necroptosis with characterized primary target.
|
|---|---|
| Targets/IC50/Ki |
IDO
RIP1
(293T cells) 490 nM(EC50)
|
| In vitro |
La Necrostatin-1 (1-100 μM) inibisce l'autofosforilazione di RIP1 sovraespresso ed endogeno. Si è riscontrato che RIP1 è il bersaglio cellulare primario responsabile dell'attività antinecroptotica di questo composto. Questa sostanza chimica sopprime efficacemente la morte cellulare necroptotica innescata da una serie di stimoli in una varietà di tipi cellulari. Essa, precedentemente identificata come inibitore a piccole molecole della necroptosi, inibisce la necroptosi indotta dalla RIP kinase e inibisce la necroptosi indotta da TNF-α nelle cellule Jurkat con una EC50 di 490 nM. |
| Saggio chinasico |
Saggio della chinasi RIP1
|
|
La fosforilazione di RIP1 richiede la sua attività chinasica. Costrutti di espressione di RIP1 wild-type (WT) taggato FLAG o di un mutante puntiforme chinasico inattivo di RIP1 (K45M) vengono trasfettati in cellule 293T e il saggio della chinasi RIP1 viene eseguito come descritto nei Metodi in presenza di [γ-32P]ATP per 30 min a 30℃. I campioni vengono sottoposti a SDS-PAGE e la banda RIP1 viene visualizzata mediante autoradiografia. Le intensità relative delle bande radioattive vengono quantificate e mostrate (rapporto) in questo e in tutti gli altri autoradiogrammi. Parallelamente alle reazioni chinasiche, un campione di perle viene sottoposto ad analisi Western blot utilizzando un anticorpo anti-RIP1 per garantire quantità uguali di proteine nelle reazioni chinasiche.
|
|
| In vivo |
Necrostatin-1 (Nec-1) è un inibitore specifico a piccole molecole della proteina chinasi 1 (RIPK1) interagente con il recettore che inibisce specificamente la fosforilazione di questo composto. |
Riferimenti |
|
Applicazioni
| Metodi | Biomarcatori | Immagini | PMID |
|---|---|---|---|
| Immunofluorescence | RIP1 / RIP3 |
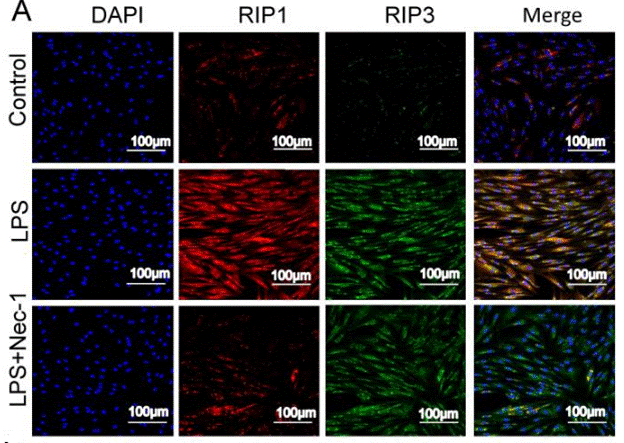
|
30462730 |
Supporto tecnico
Istruzioni per la manipolazione
Tel: +1-832-582-8158 Ext:3
Per qualsiasi altra domanda, si prega di lasciare un messaggio.
