solo per uso di ricerca
Doxycycline MMP Inhibitor
N. Cat.S5159
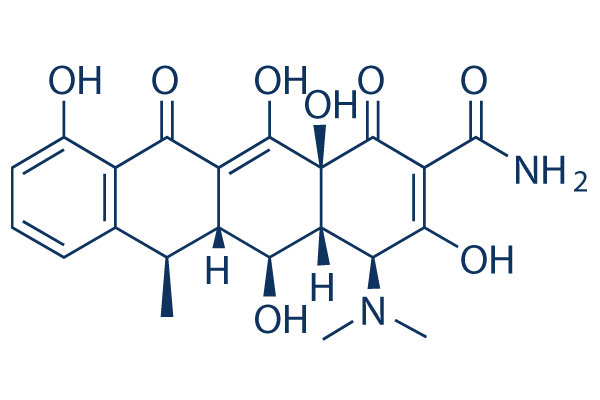
Struttura chimica
Peso molecolare: 444.43
Controllo Qualità
- Citato su Nature Medicine per la sua qualità superiore
- COA
- Scheda dati
- SDS
- Citato su Nature Medicine per la sua qualità superiore
- COA
- Scheda dati
- SDS
- Citato su Nature Medicine per la sua qualità superiore
- COA
- Scheda dati
- SDS
- Citato su Nature Medicine per la sua qualità superiore
- COA
- Scheda dati
- SDS
- Citato su Nature Medicine per la sua qualità superiore
- COA
- Scheda dati
- SDS
| Target correlati | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Altro Antineoplastic and Immunosuppressive Antibiotics Inibitori | Staurosporine (STS) Cyclosporin A Oligomycin A (MCH 32) Puromycin Dihydrochloride Nigericin sodium salt Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Sodium Monensin (NSC 343257) Cephalomannine |
Coltura cellulare, trattamento e concentrazione di lavoro
| Linee cellulari | Tipo di saggio | Concentrazione | Tempo di incubazione | Formulazione | Descrizione dellattività | PMID |
|---|---|---|---|---|---|---|
| Staphylococcus aureus 2 planktonic cells | Bactericidal assay | 24 hrs | Bactericidal activity against methicillin-resistant Staphylococcus aureus 2 planktonic cells after 24 hrs by calgary biofilm device method, MBC=2μM. | 29638121 | ||
| THP1 | Function assay | Selectivity index, ratio of IC50 for human THP1 cells to IC50 for Plasmodium falciparum, IC50=3.1μM. | 19748781 | |||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19482476 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19926173 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19482476 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19926173 | ||
| THP1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human THP1 cells after 72 hrs by propidium iodide staining-based flow cytometry, IC50=20μM. | 19748781 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21741131 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 22889559 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21852132 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 24946216 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as cell viability after 72 hrs by MTT assay, CC50=20μM. | 25791675 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells incubated for 72 hrs by MTT assay, CC50=20μM. | 25282267 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells measured after 72 hrs by MTT assay, CC50=20μM. | 27155463 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 72 hrs by MTT assay, CC50=20μM. | 27654395 | ||
| SW1353 | Function assay | 50 uM | Inhibition of IL-1-beta-induced MMP13 production in human SW1353 cells at 50 uM | 17267227 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against yafQ gene-deficient Escherichia coli K-12 BW25113 biofilm assessed as log reduction of viable cells after 24 hrs | 19307375 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against Escherichia coli K-12 BW25113 biofilm harboring pCA24N ptac::yafQ plasmid assessed as log reduction of viable cells after 24 hrs pretreated with 5 uM of IPTG for 4 hrs | 19307375 | ||
| Neuro2a | Function assay | 1 uM | Decrease in 7-DHC levels in Dhcr7-deficient mouse Neuro2a cells at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| vascular endothelial cells | Antibacterial assay | 25 ug/ml | 72 hrs | Antibacterial activity against Rickettsia prowazekii str. Breinl infected in CD rat primary pulmonary vascular endothelial cells assessed as bacterial shape change at 25 ug/ml measured 72 hrs post infection by Hoechst 33258/phalloidin staining based fluor | 28089350 | |
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Hep2 | Anti-Chlamydial assay | 1 ug/ml | 8 hrs | Anti-Chlamydial activity against Chlamydia trachomatis Serovar LGV-L2 infected in Hep2 cells assessed as reduction in size and number of chlamydial inclusion at 1 ug/ml treated at 8 hrs post-infection and 24 hrs later re-infecting fresh Hep2 cells monolay | 32227948 | |
| skeletal myoblast cells | Cytotoxicity assay | DNDI: Cytotoxicity in Vitro, 72 hour, in rat skeletal myoblast cells, IC50=14.49μM. | ChEMBL | |||
| Clicca per visualizzare più dati sperimentali sulle linee cellulari | ||||||
Informazioni chimiche, conservazione e stabilità
| Peso molecolare | 444.43 | Formula | C22H24N2O8 |
Conservazione (Dalla data di ricezione) | |
|---|---|---|---|---|---|
| N. CAS | 564-25-0 | Scarica SDF | Conservazione delle soluzioni stock |
|
|
| Sinonimi | Vibramycin, Doxytetracycline, Doxiciclina, Doxycyclinum | Smiles | CC1C2C(C3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O | ||
Solubilità
|
In vitro |
DMSO
: 89 mg/mL
(200.25 mM)
Water : Insoluble Ethanol : Insoluble |
Calcolatore di Molarità
|
In vivo |
|||||
Calcolatore di formulazione in vivo (Soluzione chiara)
Passo 1: Inserire le informazioni di seguito (Consigliato: Un animale aggiuntivo per tenere conto della perdita durante lesperimento)
Passo 2: Inserire la formulazione in vivo (Questo è solo il calcolatore, non la formulazione. Contattateci prima se non cè una formulazione in vivo nella sezione Solubilità.)
Risultati del calcolo:
Concentrazione di lavoro: mg/ml;
Metodo per preparare il liquido master di DMSO: mg farmaco predissolto in μL DMSO ( Concentrazione del liquido master mg/mL, Vi preghiamo di contattarci prima se la concentrazione supera la solubilità del DMSO del lotto del farmaco. )
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungereμL PEG300, mescolare e chiarire, quindi aggiungereμL Tween 80, mescolare e chiarire, quindi aggiungere μL ddH2O, mescolare e chiarire.
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungere μL Olio di mais, mescolare e chiarire.
Nota: 1. Si prega di assicurarsi che il liquido sia limpido prima di aggiungere il solvente successivo.
2. Assicurarsi di aggiungere il/i solvente/i in ordine. È necessario assicurarsi che la soluzione ottenuta, nellaggiunta precedente, sia una soluzione limpida prima di procedere allaggiunta del solvente successivo. Metodi fisici come il vortex, gli ultrasuoni o il bagno dacqua calda possono essere utilizzati per facilitare la dissoluzione.
Meccanismo dazione
| In vitro |
100 ng/mL-5 µg/mL Doxycycline può alterare significativamente il profilo metabolico della cellula, oltre a ridurre il tasso proliferativo, sebbene l'entità dell'effetto dipenda dalla particolare linea cellulare utilizzata. Questo composto arresta le cellule nella fase G1-S del ciclo cellulare nelle cellule PANC-1 e induce l'espressione di p53 e del suo bersaglio a valle p21. Regola al ribasso i geni anti-apoptotici e induce i geni pro-apoptotici. Questa sostanza chimica induce anche l'apoptosi attraverso una via dipendente da Fas/Fas-ligando nei linfociti T Jurkat. |
|---|---|
| In vivo |
Doxycycline riduce con successo la crescita tumorale in vivo (ha inibito la crescita delle cellule di cancro al pancreas). |
Riferimenti |
|
Applicazioni
| Metodi | Biomarcatori | Immagini | PMID |
|---|---|---|---|
| Western blot | AKT / BCL6 / Cyclin E / HDAC2 / HDAC3 / NEMO / TYK2 / RIPK1 HSP70 / HSP90 pATM / ATM |
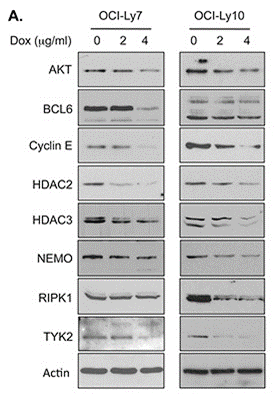
|
26142707 |
| Immunofluorescence | E-cadherin / Vimentin |
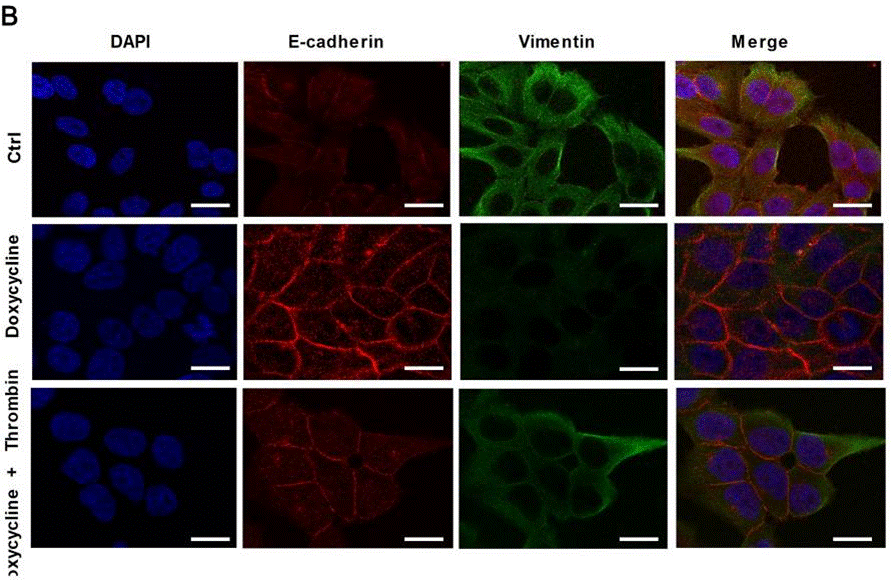
|
29285218 |
Informazioni sullo studio clinico
(dati da https://clinicaltrials.gov, aggiornato il 2024-05-22)
| Numero NCT | Reclutamento | Condizioni | Sponsor/Collaboratori | Data di inizio | Fasi |
|---|---|---|---|---|---|
| NCT05972772 | Not yet recruiting | Infectious Disease|Therapeutics |
Lao-Oxford-Mahosot Hospital Wellcome Trust Research Unit|Mahidol Oxford Tropical Medicine Research Unit |
March 20 2024 | Phase 2|Phase 3 |
| NCT06007534 | Recruiting | Post-exposure Prophylaxis|Sexually Transmitted Diseases|Doxycycline |
Assistance Publique - Hôpitaux de Paris |
October 25 2023 | Not Applicable |
| NCT04762134 | Recruiting | Bacterial Sexually Transmitted Diseases |
Jonathan Troy Grennan|Canadian Institutes of Health Research (CIHR)|British Columbia Centre for Disease Control |
June 2 2023 | Phase 4 |
| NCT05853120 | Recruiting | Sexually Transmitted Diseases |
Emory University|Centers for Disease Control and Prevention |
May 31 2023 | Phase 4 |
| NCT05382208 | Recruiting | Emphysema|HIV |
Weill Medical College of Cornell University|National Heart Lung and Blood Institute (NHLBI)|University of California Los Angeles|University of Iowa|University of Michigan |
August 22 2022 | Phase 2 |
| NCT05492019 | Recruiting | Parkinson Disease |
Bangabandhu Sheikh Mujib Medical University Dhaka Bangladesh |
July 1 2022 | Phase 2 |
Supporto tecnico
Istruzioni per la manipolazione
Tel: +1-832-582-8158 Ext:3
Per qualsiasi altra domanda, si prega di lasciare un messaggio.
