solo per uso di ricerca
Berberine chloride Anti-infection chimico
N. Cat.S2271
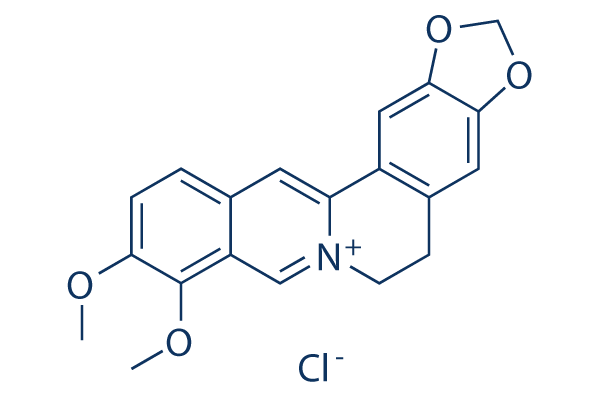
Struttura chimica
Peso molecolare: 371.81
Controllo Qualità
Coltura cellulare, trattamento e concentrazione di lavoro
| Linee cellulari | Tipo di saggio | Concentrazione | Tempo di incubazione | Formulazione | Descrizione dellattività | PMID |
|---|---|---|---|---|---|---|
| MRC5 cells | Function assay | Antiviral activity against HCMV in MRC5 cells by plaque reduction assay, IC50=0.68 μM | ||||
| MRC5 cells | Proliferation assay | 10 μM | 54 hrs | Inhibition of HCMV proliferation in MRC5 cells after 54 hrs post-infection at 10 uM by plaque assay | ||
| MRC5 cells | Proliferation assay | 10 μM | 24 h | Inhibition of HCMV proliferation in MRC5 cells after 24 hrs post-infection at 10 uM by plaque assay | ||
| Bel7402 cells | Function assay | 12 h | Induction of LDLR protein in human Bel7402 cells after 12 hrs by RT-PCR assay relative to control | |||
| HepG2 cells | Function assay | 10 ug/mL | 12 h | Induction of LDLR protein expression in human HepG2 cells at 10 ug/mL after 12 hrs by flow cytometry | ||
| KB cells | Cytotoxicity assay | 72 h | Cytotoxicity against human KB cells after 72 hrs, IC50=7.32 μM | |||
| HL60 cells | Apoptosis assay | 48 hrs | Induction of apoptosis in human HL60 cells after 48 hrs using annexin V-propidium iodide staining by FACS analysis | |||
| A549 cells | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB assay, IC50=6.27 μM | ||||
| SKOV3 cells | Cytotoxicity assay | Cytotoxicity against human SKOV3 cells by SRB assay, IC50=16.44 μM | ||||
| SK-MEL-2 cells | Cytotoxicity assay | Cytotoxicity against human SK-MEL-2 cells by SRB assay, IC50=13.76 μM | ||||
| HCT15 cells | Cytotoxicity assay | Cytotoxicity against human HCT15 cells by SRB assay, IC50=16.59 μM | ||||
| CEM cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CEM cells expressing green fluorescent protein after 48 hrs by MTT assay, CC50=2.09 μM | |||
| human CEM cells | Function assay | 7 days | Antiviral activity against 0.05 MOI Human immunodeficiency virus 1 NL4.3 infected in human CEM cells expressing green fluorescent protein assessed as p24 antigen production measured 7 days post infection by ELISA, EC50=0.13 μM | |||
| SKN cells | Growth inhibition assay | 72 h | Growth inhibition against human SKN cells after 72 hrs by MTT assay, GI50=15.88 μM | |||
| RKN cells | Growth inhibition assay | 48 hrs | Growth inhibition against human RKN cells after 48 hrs by MTT assay, GI50=49.6 μM | |||
| G402 cells | Growth inhibition assay | 48 hrs | Growth inhibition against human G402 cells after 48 hrs by MTT assay, GI50=11.87 μM | |||
| A10 cells | Function assay | 30 μM | 24 hrs | Downregulation of Scd2 mRNA expression in rat A10 cells at 30 uM after 24 hrs by quantitative RT-PCR analysis | ||
| A10 cells | Function assay | 30 μM | 24 hrs | Down regulation of Prim2 mRNA expression in rat A10 cells at 30 uM after 24 hrs by quantitative RT-PCR analysis | ||
| A10 cells | Function assay | 30 μM | 24 hrs | Downregulation of Impk mRNA expression in rat A10 cells at 30 uM after 24 hrs by quantitative RT-PCR analysis | ||
| HepG2-A16-CD81 cells | Function assay | 10 μM | NOVARTIS: Antimalarial liver stage activity measured as a greater than 50% reduction in Plasmodium yoelii schizont area in HepG2-A16-CD81 cells at 10uM compound concentration, determined by immuno-fluorescence. | |||
| HepG2-A16-CD81 cells | Function assay | 10 μM | NOVARTIS: Antimalarial liver stage activity measured as reduction in Plasmodium yoelii schizont area in HepG2-A16-CD81 cells by immuno-fluorescence, and median schizont size at 10uM compound concentration, IC50=0.548 μM | |||
| HepG2 cells | Function assay | 10 μM | 4 h | Increase in AMPKalpha phosphorylation in human HepG2 cells at 10 uM after 4 hrs by Western blot analysis relative to untreated control | ||
| HepG2 cells | Function assay | 10 μM | 4 h | Increase in total AMPKalpha level in human HepG2 cells at 10 uM after 4 hrs by Western blot analysis relative to untreated control | ||
| HepG2 cells | Function assay | 20 μM | 24 hrs | Induction of apoptosis in human HepG2 cells assessed as morphological changes at 20 uM after 24 hrs using Hoechst 33258 staining by fluorescence microscopic analysis | ||
| HT-29 cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells after 48 hrs by MTT assay, IC50=8.45 μM | |||
| HepG2 cells | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells after 24 hrs by MTT assay, IC50=11.22 μM | |||
| HepG2 cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50=8.32 μM | |||
| Clicca per visualizzare più dati sperimentali sulle linee cellulari | ||||||
Informazioni chimiche, conservazione e stabilità
| Peso molecolare | 371.81 | Formula | C20H18NO4.Cl |
Conservazione (Dalla data di ricezione) | |
|---|---|---|---|---|---|
| N. CAS | 633-65-8 | Scarica SDF | Conservazione delle soluzioni stock |
|
|
Solubilità
|
In vitro |
DMSO
: 25 mg/mL
(67.23 mM)
Water : Insoluble Ethanol : Insoluble |
Calcolatore di Molarità
|
In vivo |
|||||
Calcolatore di formulazione in vivo (Soluzione chiara)
Passo 1: Inserire le informazioni di seguito (Consigliato: Un animale aggiuntivo per tenere conto della perdita durante lesperimento)
Passo 2: Inserire la formulazione in vivo (Questo è solo il calcolatore, non la formulazione. Contattateci prima se non cè una formulazione in vivo nella sezione Solubilità.)
Risultati del calcolo:
Concentrazione di lavoro: mg/ml;
Metodo per preparare il liquido master di DMSO: mg farmaco predissolto in μL DMSO ( Concentrazione del liquido master mg/mL, Vi preghiamo di contattarci prima se la concentrazione supera la solubilità del DMSO del lotto del farmaco. )
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungereμL PEG300, mescolare e chiarire, quindi aggiungereμL Tween 80, mescolare e chiarire, quindi aggiungere μL ddH2O, mescolare e chiarire.
Metodo per preparare la formulazione in vivo: Prendere μL DMSO liquido master, quindi aggiungere μL Olio di mais, mescolare e chiarire.
Nota: 1. Si prega di assicurarsi che il liquido sia limpido prima di aggiungere il solvente successivo.
2. Assicurarsi di aggiungere il/i solvente/i in ordine. È necessario assicurarsi che la soluzione ottenuta, nellaggiunta precedente, sia una soluzione limpida prima di procedere allaggiunta del solvente successivo. Metodi fisici come il vortex, gli ultrasuoni o il bagno dacqua calda possono essere utilizzati per facilitare la dissoluzione.
Meccanismo dazione
| Targets/IC50/Ki |
Caspase-3
Caspase-8
PARP
cytochrome c
cIAP1
Bcl-2
Bcl-xL
JNK
p38 MAPK
ROS
Topo I
Topo II
|
|---|---|
| In vitro |
Rispetto al regorafenib da solo, il trattamento combinato di Berberine (BBR) e regorafenib inibisce significativamente la proliferazione delle cellule di carcinoma epatocellulare (HCC) e induce l'apoptosis cellulare. |
| In vivo |
Il gruppo di trattamento combinato con Berberine (BBR) e regorafenib ha un effetto inibitorio drammatico sulla crescita dei tumori di xenotrapianto di carcinoma epatocellulare (HCC) in topi nudi. L'aumentata apoptosis dei tumori di xenotrapianto si osserva nel gruppo di trattamento combinato. |
Riferimenti |
|
Informazioni sullo studio clinico
(dati da https://clinicaltrials.gov, aggiornato il 2024-05-22)
| Numero NCT | Reclutamento | Condizioni | Sponsor/Collaboratori | Data di inizio | Fasi |
|---|---|---|---|---|---|
| NCT06273241 | Not yet recruiting | Pharmacokinetic Study in Healthy Volunteers |
University Medicine Greifswald |
March 4 2024 | Not Applicable |
| NCT05845931 | Recruiting | Pharmacokinetic Study in Healthy Volunteers |
University Medicine Greifswald |
May 5 2023 | Not Applicable |
| NCT05480670 | Completed | Polycystic Ovary Syndrome |
Ayub Teaching Hospital |
November 1 2022 | Not Applicable |
| NCT05463003 | Completed | Pharmacokinetic Study in Healthy Volunteers |
University Medicine Greifswald |
July 19 2022 | Not Applicable |
Supporto tecnico
Istruzioni per la manipolazione
Tel: +1-832-582-8158 Ext:3
Per qualsiasi altra domanda, si prega di lasciare un messaggio.
