réservé à la recherche
Avapritinib (BLU-285) inhibiteur de PDGFRα
N° Cat.S8553
Structure chimique
Poids moléculaire: 498.56
Contrôle qualité
| Cibles apparentées | EGFR VEGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Autre PDGFR Inhibiteurs | CP-673451 Orantinib (SU6668) Tyrphostin AG 1296 Trapidil PP121 AZD2932 Sennoside B Tyrphostin AG1433 Seralutinib (GB002) N-(p-Coumaroyl) Serotonin |
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit D816V mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.008 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of PDGFRalpha V561D/D842V mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.01 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (557 to 558 residues) and D816H mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.017 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (557 to 558 residues) and D820A mutant and D820A mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.019 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of TEL-fused PDGFRbeta (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.022 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (557 to 558 residues) and A829P mutant and Y823D mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.028 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit V560D/D816H mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.029 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of TEL-fused PDGFRalpha (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.034 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (557 to 558 residues) and N822K mutant and Y823D mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.034 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit V560D mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.042 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 9 AY502 to 503 insertion and D816 mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.069 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (557 to 558 residues) mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.078 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (560 to 578 residues) mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.117 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 9 AY502 to 503 insertion mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.167 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (557 to 558 residues) and V654A mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.292 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit V560D/V654A mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.427 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 9 AY502 to 503 insertion and V654 mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.751 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of Kit exon 11 deletion (557 to 558 residues) and D816H mutant and T670I mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.86 μM. | 30204441 | ||
| BA/F3 cells | Cytotoxicity assay | 72 h | Cytotoxicity in mouse parental BA/F3 cells incubated for 72 hrs by MTS assay, GI50 = 4.075 μM. | 30204441 | ||
| BA/F3 cells | Growth inhibiton assay | 72 h | Inhibition of KDR (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 4.952 μM. | 30204441 | ||
| Cliquez pour voir plus de données expérimentales sur les lignées cellulaires | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 498.56 | Formule | C26H27FN10 |
Stockage (À partir de la date de réception) | |
|---|---|---|---|---|---|
| N° CAS | 1703793-34-3 | Télécharger le SDF | Stockage des solutions mères |
|
|
| Synonymes | BLU-285 | Smiles | CC(C1=CC=C(C=C1)F)(C2=CN=C(N=C2)N3CCN(CC3)C4=NC=NN5C4=CC(=C5)C6=CN(N=C6)C)N | ||
Solubilité
|
In vitro |
DMSO
: 100 mg/mL
(200.57 mM)
Ethanol : 1.5 mg/mL Water : Insoluble |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Entrez les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Entrez la formulation in vivo (Ceci nest que le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, ajouter ensuiteμL PEG300, mélanger et clarifier, ajouter ensuiteμL Tween 80, mélanger et clarifier, ajouter ensuite μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, ajouter ensuite μL Huile de maïs, mélanger et clarifier.
Remarque : 1. Assurez-vous que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue lors de lajout précédent est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
PDGFRα (D842V)
0.5 nM
c-Kit (D816V)
0.5 nM
|
|---|---|
| In vitro |
Avapritinib (BLU-285) est un inhibiteur oral sélectif qui cible les mutants de l'exon 17 de KIT et de la boucle d'activation PDGFRα D842. Des essais cellulaires mesurant l'inhibition de l'autophosphorylation du mutant KIT confirment son activité contre les mutants KIT D816 D816V (cellules HMC1.2, IC50 = 3 nM) et D816Y (cellules P815, IC50 = 22 nM) ainsi que d'autres mutants de l'exon 17 de KIT tels que N822K (cellules Kasumi, IC50 = 40 nM) trouvés dans les GIST réfractaires au traitement. |
| In vivo |
In vivo, Avapritinib (BLU-285) est un agent bien toléré, biodisponible par voie orale, qui entraîne une inhibition de la croissance tumorale dose-dépendante dans un modèle de xénogreffe piloté par D816Y. Une relation PK-PD-efficacité a été établie avec ce composé, démontrant que la régression tumorale résulte d'une suppression de la cible >90 % et est observée avec une dose de 30 mg/kg une fois par jour. Grâce à son activité puissante contre les mutants PDGFRα D842V et KIT Exon 17, il cible des moteurs génomiques de la maladie précédemment non traités et offre des promesses pour le traitement des GIST (tumeur stromale gastro-intestinale) ou SM (mastocytose systémique) pilotées par PDGFRα D842V, où plus de 90 % des patients portent la mutation KIT D816V. Outre l'activité en monothérapie, le BLU-285 hautement sélectif offre une opportunité de combinaison avec d'autres agents dans les GIST pour couvrir l'ensemble des mutants primaires et de résistance de KIT. |
Références |
|
Applications
| Méthodes | Biomarqueurs | Images | PMID |
|---|---|---|---|
| Western blot | PDGFRA / c-KIT / Wee1 / Cdc2 / PARP / γH2AX / CyclinD1 / β-actin |
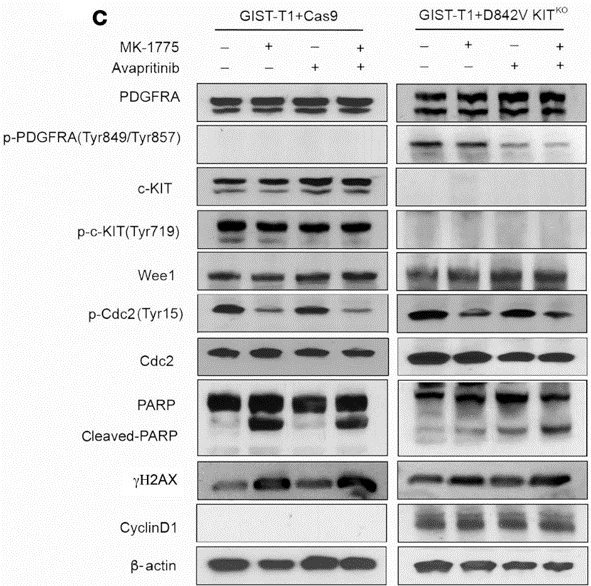
|
33320833 |
Informations sur lessai clinique
(données de https://clinicaltrials.gov, mis à jour le 2024-05-22)
| Numéro NCT | Recrutement | Conditions | Sponsor/Collaborateurs | Date de début | Phases |
|---|---|---|---|---|---|
| NCT04908176 | Completed | Gastrointestinal Stromal Tumors|GIST|Non-resectable Advanced Solid Tumors|Recurrent or Unresectable Central Nervous System (CNS) Tumors |
Blueprint Medicines Corporation |
August 24 2022 | Phase 1 |
| NCT04695431 | Completed | Advanced Systemic Mastocytosis|Aggressive Systemic Mastocytosis|Systemic Mastocytosis With an Associated Hematological Neoplasm|Mast Cell Leukemia |
Blueprint Medicines Corporation|Analysis Group Inc. |
December 2 2020 | -- |
| NCT03731260 | Active not recruiting | Indolent Systemic Mastocytosis |
Blueprint Medicines Corporation |
April 16 2019 | Phase 2 |
| NCT03580655 | Active not recruiting | Advanced Systemic Mastocytosis|Aggressive Systemic Mastocytosis|Systemic Mastocytosis With an Associated Hematologic Neoplasm|Mast Cell Leukemia |
Blueprint Medicines Corporation |
November 21 2018 | Phase 2 |
| NCT03465722 | Completed | GIST |
Blueprint Medicines Corporation |
March 26 2018 | Phase 3 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
