réservé à la recherche
Nitazoxanide Influenza Virus inhibiteur
N° Cat.S1627
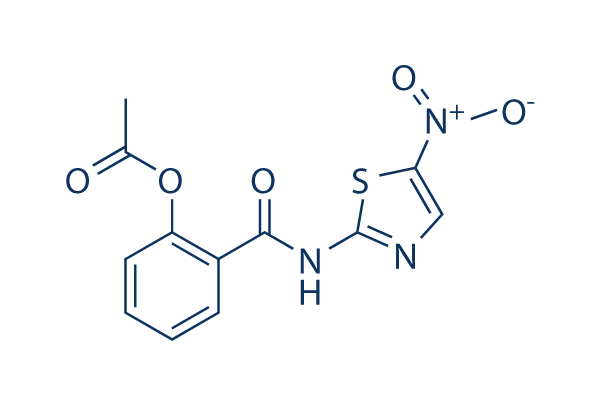
Structure chimique
Poids moléculaire: 307.28
Contrôle qualité
| Cibles apparentées | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Autre Influenza Virus Inhibiteurs | Arbidol HCl Nucleozin D-Pinitol (-)-Arctigenin Adamantane Cetylpyridinium chloride monohydrate Chebulinic acid |
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as decrease in extracellular viral DNA measured 24 hrs after last dose, EC50=0.12μM | 21553812 | |||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against HBV infected in human HepG2(2.2.15) cells assessed as inhibition of extracellular viral DNA level, IC50=0.12μM | 28383274 | |||
| Ava5 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in Ava5 cells assessed as inhibition of viral replication after 3 days by blot hybridization analysis, EC50=0.21μM | 22059983 | ||
| Huh7.5 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1a infected in Huh7.5 cells assessed as inhibition of viral replication after 3 days by blot hybridization analysis, EC50=0.33μM | 22059983 | ||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as decrease in intracellular viral DNA measured 24 hrs after last dose, EC50=0.59μM | 21553812 | |||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as decrease in extracellular viral DNA measured 24 hrs after last dose, EC90=0.83μM | 21553812 | |||
| Ava5 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in Ava5 cells assessed as inhibition of viral replication after 3 days by blot hybridization analysis, EC90=0.93μM | 22059983 | ||
| Huh7.5 | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1a infected in Huh7.5 cells assessed as inhibition of viral replication after 3 days by blot hybridization analysis, EC90=1.1μM | 22059983 | ||
| BHK-21 | Antiviral assay | Antiviral activity against Chikungunya virus 0611aTw infected in BHK-21 cells by RT-qPCR analysis, EC50=1.96μM | 28689975 | |||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as decrease in intracellular viral DNA measured 24 hrs after last dose, EC90=2.1μM | 21553812 | |||
| HCT8 | Antiparasitic assay | 48 hrs | Antiparasitic activity against Cryptosporidium parvum SPL infected in human HCT8 cells incubated for 48 hrs by FITC/DAPI staining based fluorescence assay, EC50=2.3μM | 29469575 | ||
| Vero E6 | Function assay | 48 hrs | IC50 for antiviral activity against SARS-CoV-2 in the Vero E6 cell line at 48 h by immunofluorescence-based assay (detecting the viral NP protein in the nucleus of the Vero E6 cells), IC50=2.81838μM | 32353859 | ||
| HCT8 | Antiparasitic assay | 48 hrs | Antiparasitic activity against Cryptosporidium parvum BGF infected in human HCT8 cells incubated for 48 hrs by FITC/DAPI staining based fluorescence assay, EC50=2.9μM | 29469575 | ||
| BHK-21 | Antiviral assay | Antiviral activity against Chikungunya virus infected in BHK-21 cells by RT-qPCR analysis, EC50=2.96μM | 28689975 | |||
| U2OS | Antiviral assay | Antiviral activity against Chikungunya virus infected in human U2OS cells by RT-qPCR analysis, EC50=3.01μM | 28689975 | |||
| HCT8 | Antiparasitic assay | Antiparasitic activity against Cryptosporidium parvum in HCT8 cells, IC50=3.25μM | 16480281 | |||
| HCT-8 | Antimicrobial assay | Antimicrobial activity against Cryptosporidium parvum infected in human HCT-8 cells, IC50=3.8μM | 18591280 | |||
| BHK-21 | Antiviral assay | Antiviral activity against Chikungunya virus 0810bTw infected in BHK-21 cells by RT-qPCR analysis, EC50=4.95μM | 28689975 | |||
| Vero | Cytotoxicity assay | 48 hrs | Cytotoxicity against african green monkey Vero cells assessed as cell viability after 48 hrs by WST-1 assay, IC50=10.74μM | 23787289 | ||
| BHK21 | Cytotoxicity assay | 16 hrs | Cytotoxicity against BHK21 cells assessed as reduction in cell viability after 16 hrs by CCK-8 assay, CC50=25μM | 28689975 | ||
| U2OS | Cytotoxicity assay | 16 hrs | Cytotoxicity against human U2OS cells assessed as reduction in cell viability after 16 hrs by CCK-8 assay, CC50=25μM | 28689975 | ||
| Ava5 | Cytotoxicity assay | 3 days | Cytotoxicity against human Ava5 cells after 3 days by neutral red dye assay, CC50=35μM | 22059983 | ||
| Huh7.5 | Cytotoxicity assay | 3 days | Cytotoxicity against human Huh7.5 cells after 3 days by neutral red dye assay, CC50=49μM | 22059983 | ||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| Cliquez pour voir plus de données expérimentales sur les lignées cellulaires | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 307.28 | Formule | C12H9N3O5S |
Stockage (À partir de la date de réception) | |
|---|---|---|---|---|---|
| N° CAS | 55981-09-4 | Télécharger le SDF | Stockage des solutions mères |
|
|
| Synonymes | NTZ, NSC 697855 | Smiles | CC(=O)OC1=CC=CC=C1C(=O)NC2=NC=C(S2)[N+](=O)[O-] | ||
Solubilité
|
In vitro |
DMSO
: 62 mg/mL
(201.77 mM)
Ethanol : 62 mg/mL Water : Insoluble |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Entrez les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Entrez la formulation in vivo (Ceci nest que le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, ajouter ensuiteμL PEG300, mélanger et clarifier, ajouter ensuiteμL Tween 80, mélanger et clarifier, ajouter ensuite μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, ajouter ensuite μL Huile de maïs, mélanger et clarifier.
Remarque : 1. Assurez-vous que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue lors de lajout précédent est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
PFOR
mTORC1
|
|---|---|
| In vitro |
Le Nitazoxanide réduit la croissance parasitaire en culture cellulaire de plus de 90 % avec peu de preuves de cytotoxicité associée au médicament. Ce composé est un nouvel agent antiparasitaire thiazolide qui présente une excellente activité in vitro contre une grande variété de protozoaires et d'helminthes. Lui et son métabolite, le tizoxanide, sont plus actifs in vitro que le métronidazole contre G. intestinalis, E. histolytica et T. vaginalis. Cet agent exerce une puissante inhibition de la réplication du VHB et du VHC. Il potentialise l'effet d'un traitement ultérieur avec ce composé plus l'IFN, mais pas ce produit chimique plus le 2'CmeC, dans les cellules contenant le réplicon VHC. Ce produit chimique induit des réductions de plusieurs protéines du VHB (AgHBs, AgHBe, AgHBc) produites par les cellules 2.2.15, mais n'affecte pas la transcription de l'ARN du VHB. Il présente des valeurs de CI50 et de CI90 de 0,017 et 0,776 mg/mL respectivement, contre E. histolytica, 0,004 et 0,067 mg/mL contre G. intestinalis, et 0,034 et 2,046 mg/mL contre T. vaginalis. Ce composé est plus toxique que le métronidazole et l'albendazole contre E. histolytica. |
| In vivo |
Le Nitazoxanide est partiellement efficace pour réduire la charge parasitaire dans un modèle de diarrhée de porcelet gnotobiotique lorsqu'il est administré par voie orale pendant 11 jours à 250 mg/kg/jour mais pas à 125 mg/kg/jour. Ce composé induit une diarrhée liée au médicament chez les porcelets qui aurait pu influencer son efficacité thérapeutique. |
Références |
|
Informations sur lessai clinique
(données de https://clinicaltrials.gov, mis à jour le 2024-05-22)
| Numéro NCT | Recrutement | Conditions | Sponsor/Collaborateurs | Date de début | Phases |
|---|---|---|---|---|---|
| NCT06049901 | Recruiting | Metastatic Colorectal Cancer |
Tanta University |
March 1 2023 | Phase 3 |
| NCT05701423 | Recruiting | Multiple Sclerosis |
Biogen |
February 8 2023 | -- |
| NCT05368935 | Completed | Renal Impairment|Renal Disease|Kidney Disease |
Genfit |
April 25 2022 | Phase 1 |
| NCT05116826 | Completed | Moderate Hepatic Impairment|Severe Hepatic Impairment|Liver Diseases |
Genfit |
November 5 2021 | Phase 1 |
| NCT03656068 | Completed | Non-alcoholic Steatohepatitis|Fatty Liver|Fibrosis Liver|Compensated Cirrhosis |
Pinnacle Clinical Research PLLC |
December 4 2018 | Phase 2 |
| NCT02684240 | Completed | Tuberculosis |
Weill Medical College of Cornell University |
February 2016 | Phase 2 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
